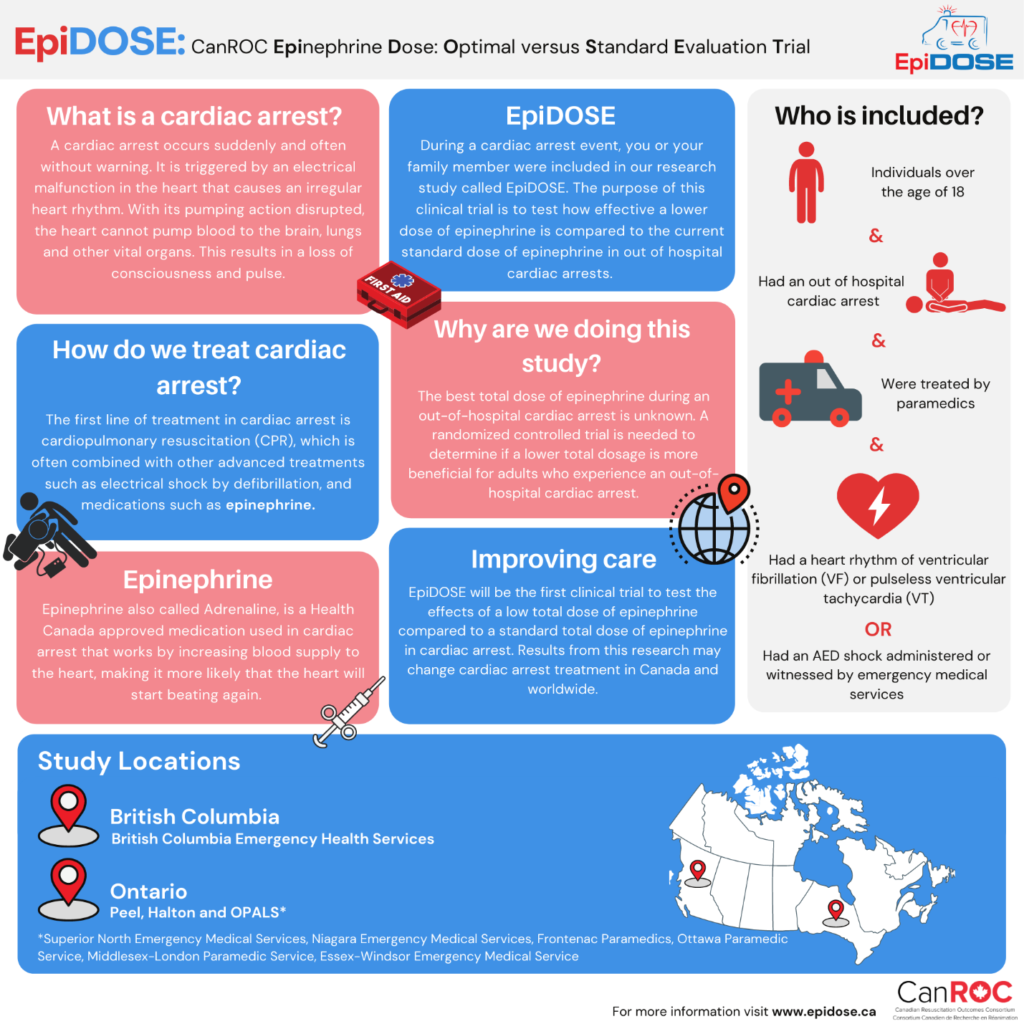
During the cardiac arrest event, you or your family member were included in our research study called EpiDOSE. EpiDOSE is a study to test if a lower dose of epinephrine has more benefits for patients compared to the current standard dose of epinephrine which is typically administered during CPR. At the time of your cardiac arrest event, paramedics gave either a standard dose or a lower dose of epinephrine and collected data to answer this question. As you were not conscious or breathing when you had your event we could not ask for your permission to collect this data, as a result, this was collected under a waiver of consent (without your permission) to allow us access to your ambulance and hospital records.
If you have any questions or would like to talk with a study team member about the trial, please contact us at epidose@unityhealth.to