About EpiDOSE
Each year in Canada, there are over 40,000 people who collapse from cardiac arrest. Sudden cardiac arrest happens when the heart suddenly and unexpectedly stops beating, reducing blood flow to the brain and other vital organs. Causes of cardiac arrest include dangerous and irregular heart rhythms that cause blockages in the arteries that supply blood to the heart, as well as other causes that do not affect the heart like illegal drugs or motor vehicle accidents.
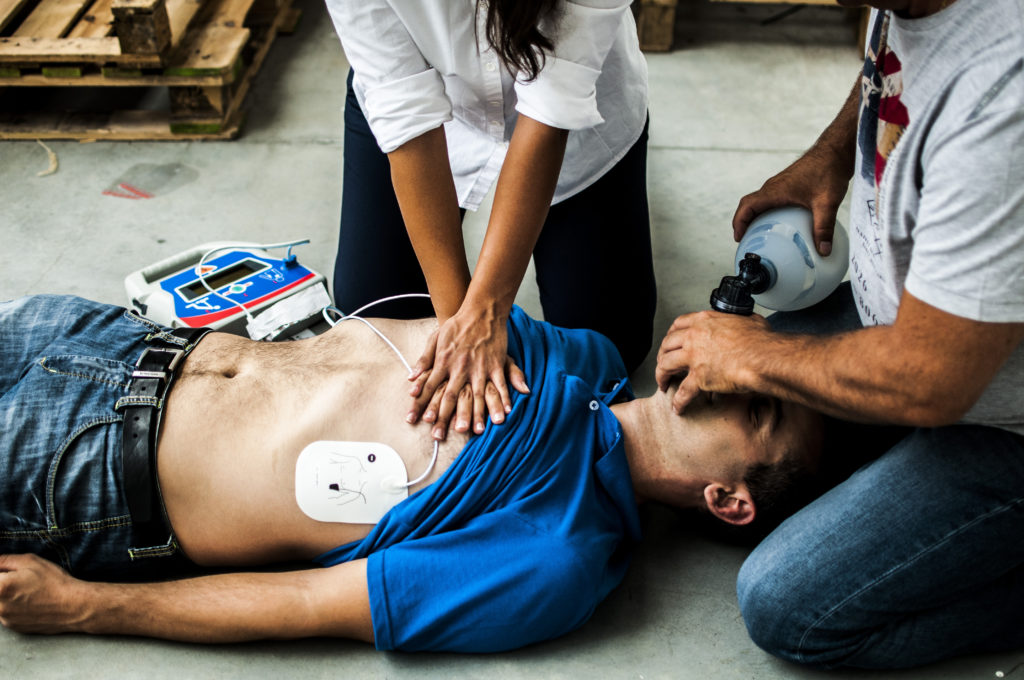
This life-threatening event is treated by paramedics with cardiopulmonary resuscitation, defibrillator shocks, and drugs, including the routine use of a medication called epinephrine (adrenaline). Epinephrine can help reverse cardiac arrest by increasing blood flow to the heart and other organs. In Canada, the standard dose given by paramedics is 1mg of epinephrine every 3 to 5 minutes. Although the standard dose of epinephrine can help restart the heart in the short term, many patients will not survive to leave the hospital from causes like irreversible brain injury and brain death.
Even though epinephrine has been used for decades, there is limited proof that the standard dose of epinephrine is effective in the long term at improving long-term outcomes such as survival following hospital discharge. There has been some research to suggest that a lower dose of epinephrine may improve long-term outcomes, but more research is needed to determine this.
The objective
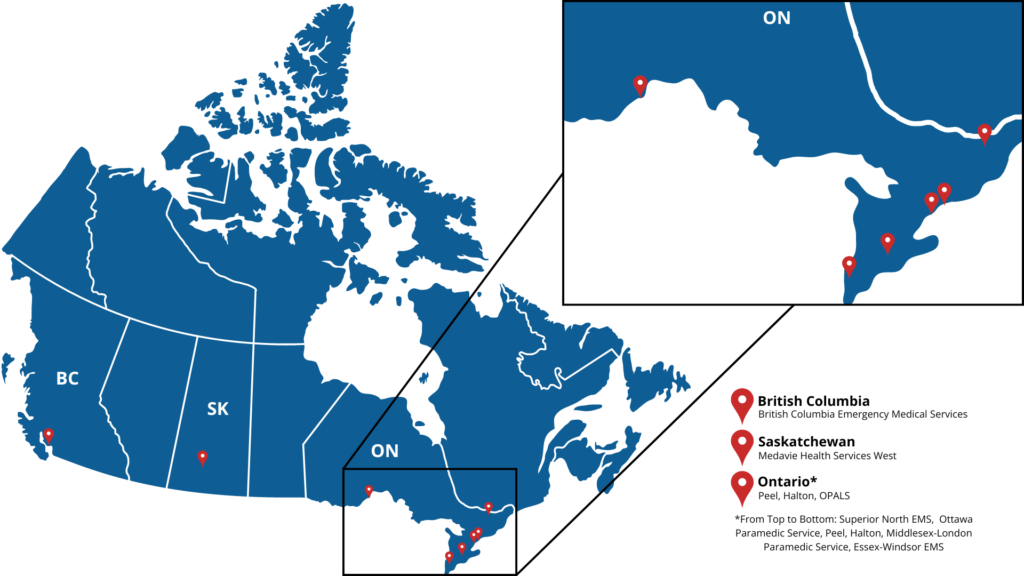
The objective of the EpiDOSE clinical trial is to improve patient outcomes by evaluating how we currently use epinephrine during resuscitation. The EpiDOSE trial will test how effective a lower dose of epinephrine is (up to 2 doses or 2mg total) compared to the current standard dose of epinephrine (up to 6 doses or 6mg total) on survival to hospital discharge in adults who experience an out-of-hospital cardiac arrest. The study will involve Emergency Medical Service agencies in British Columbia and Ontario.
EpiDOSE will be the first clinical trial to test the effects of a low total dose of epinephrine compared to a standard total dose of epinephrine in cardiac arrest. There has been some research to suggest that a lower dose of epinephrine may improve long-term outcomes, but more research is needed to determine this. Results from this research may change cardiac arrest treatment in Canada and worldwide.
Study Participation
This trial includes adults aged 18 years and older, who experience an out-of-hospital cardiac arrest from irregular heart rhythms of ventricular fibrillation and pulseless ventricular tachycardia in areas serviced by participating emergency medical services agencies.
If you or your loved one was enrolled in EpiDOSE visit here.